Density matrix plot
An intuitive way to look at eigenstates is to plot the charge density. For multi-electron wave-functions one can not plot the wave-function (it depends on $3\times n$ coordinates) but the charge density is a well defined simple quantity. We here use the rendering options of \textit{Mathematica} in combination with the package for \textit{Mathematica} (Quanty.nb) to plot the density matrix of the lowest three eigenstates in NiO.
- density_matrix_plots_Mathematica.Quanty
-- It is often nice to make plots of the resulting "wave-functions" Now one can not -- simply plot a multi-electron wavefunction \psi(r_1,r_2,r_3, ..., r_n) as it depends -- on 3n coordinates. -- One can plot the resulting charge density. -- This example calculates the density matrix of a state and then calls Mathematica to -- make a density plot of this matrix. -- the script for mathematica is included in the input file -- the input string needs calculated data which will be substituted before calling mathematica -- You need to change the absolute path in the script below mathematicaInput = [[ Needs["Quanty`PlotTools`"]; rho=%s; pl = Table[ Rasterize[ DensityMatrixPlot[IdentityMatrix[10] - rho[ [i] ], PlotRange -> {{-0.4, 0.4}, {-0.4, 0.4}, {-0.4, 0.4}}] ], {i, 1, Length[rho]}]; For[i = 1, i <= Length[pl], i++, Export["/Users/haverkort/Documents/Quanty/Example_and_Testing/History/current/Tutorials/40_NiO_Ligand_Field/Rho" <> ToString[i] <> ".png", pl[ [i] ] ]; ]; Quit[]; ]] -- We use the definitions of all operators and basis orbitals as defined in the file -- include and can afterwards directly continue by creating the Hamiltonian -- and calculating the spectra dofile("Include.Quanty") -- The parameters and scheme needed only includes the ground-state (d^8) configuration -- We follow the energy definitions as introduced in the group of G.A. Sawatzky (Groningen) -- J. Zaanen, G.A. Sawatzky, and J.W. Allen PRL 55, 418 (1985) -- for parameters of specific materials see -- A.E. Bockquet et al. PRB 55, 1161 (1996) -- After some initial discussion the energies U and Delta refer to the center of a configuration -- The L^10 d^n configuration has an energy 0 -- The L^9 d^n+1 configuration has an energy Delta -- The L^8 d^n+2 configuration has an energy 2*Delta+Udd -- -- If we relate this to the onsite energy of the L and d orbitals we find -- 10 eL + n ed + n(n-1) U/2 == 0 -- 9 eL + (n+1) ed + (n+1)n U/2 == Delta -- 8 eL + (n+2) ed + (n+1)(n+2) U/2 == 2*Delta+U -- 3 equations with 2 unknowns, but with interdependence yield: -- ed = (10*Delta-nd*(19+nd)*U/2)/(10+nd) -- eL = nd*((1+nd)*Udd/2-Delta)/(10+nd) -- -- note that ed-ep = Delta - nd * U and not Delta -- note furthermore that ep and ed here are defined for the onsite energy if the system had -- locally nd electrons in the d-shell. In DFT or Hartree Fock the d occupation is in the end not -- nd and thus the onsite energy of the Kohn-Sham orbitals is not equal to ep and ed in model -- calculations. -- -- note furthermore that ep and eL actually should be different for most systems. We happily ignore this fact -- -- We normally take U and Delta as experimentally determined parameters -- number of electrons (formal valence) nd = 8 -- parameters from experiment (core level PES) Udd = 7.3 Delta = 4.7 -- parameters obtained from DFT (PRB 85, 165113 (2012)) F2dd = 11.14 F4dd = 6.87 F2pd = 6.67 tenDq = 0.56 tenDqL = 1.44 Veg = 2.06 Vt2g = 1.21 zeta_3d = 0.081 Bz = 0.000001 H112 = 0.120 ed = (10*Delta-nd*(19+nd)*Udd/2)/(10+nd) eL = nd*((1+nd)*Udd/2-Delta)/(10+nd) F0dd = Udd + (F2dd+F4dd) * 2/63 Hamiltonian = F0dd*OppF0_3d + F2dd*OppF2_3d + F4dd*OppF4_3d + zeta_3d*Oppldots_3d + Bz*(2*OppSz_3d + OppLz_3d) + H112 * (OppSx_3d+OppSy_3d+2*OppSz_3d)/sqrt(6) + tenDq*OpptenDq_3d + tenDqL*OpptenDq_Ld + Veg * OppVeg + Vt2g * OppVt2g + ed * OppN_3d + eL * OppN_Ld -- we now can create the lowest Npsi eigenstates: Npsi=3 -- in order to make sure we have a filling of 8 electrons we need to define some restrictions StartRestrictions = {NF, NB, {"000000 00 1111111111 0000000000",8,8}, {"111111 11 0000000000 1111111111",18,18}} psiList = Eigensystem(Hamiltonian, StartRestrictions, Npsi) oppList={Hamiltonian, OppSsqr, OppLsqr, OppJsqr, OppSx_3d, OppLx_3d, OppSy_3d, OppLy_3d, OppSz_3d, OppLz_3d, Oppldots_3d, OppF2_3d, OppF4_3d, OppNeg_3d, OppNt2g_3d, OppNeg_Ld, OppNt2g_Ld, OppN_3d} -- print of some expectation values print(" # <E> <S^2> <L^2> <J^2> <S_x^3d> <L_x^3d> <S_y^3d> <L_y^3d> <S_z^3d> <L_z^3d> <l.s> <F[2]> <F[4]> <Neg^3d> <Nt2g^3d><Neg^Ld> <Nt2g^Ld><N^3d>"); for i = 1,#psiList do io.write(string.format("%3i ",i)) for j = 1,#oppList do expectationvalue = Chop(psiList[i]*oppList[j]*psiList[i]) io.write(string.format("%8.3f ",expectationvalue)) end io.write("\n") end io:flush() -- we now make plots of the charge density function tableToMathematica(t) local ret = "{ " for k,v in pairs(t) do if k~=1 then ret = ret.." , " end if (type(v) == "table") then ret = ret..tableToMathematica(v) else ret = ret..string.format("%18.15f + (%18.15f) I ",Complex.Re(v),Complex.Im(v)) end end ret = ret.." }" return ret end -- the d-orbitals are found at position 8 to 17 (see the include file) rhoList = DensityMatrix(psiList, {8,9,10,11,12,13,14,15,16,17}) rhoListMathematicaForm = tableToMathematica(rhoList) file = io.open("densitymatrix.nb", "w") file:write( mathematicaInput:format( rhoListMathematicaForm ) ) file:close() os.execute("/Applications/Mathematica08.app/Contents/MacOS/MathKernel -run '<<densitymatrix.nb'") os.execute("convert Rho1.png -transparent white temp.png ; convert temp.png Rho1.eps ; rm temp.png") os.execute("convert Rho2.png -transparent white temp.png ; convert temp.png Rho2.eps ; rm temp.png") os.execute("convert Rho3.png -transparent white temp.png ; convert temp.png Rho3.eps ; rm temp.png")
| $S_z=-1$ | $S_z=0$ | $S_z=1$ |
|---|---|---|
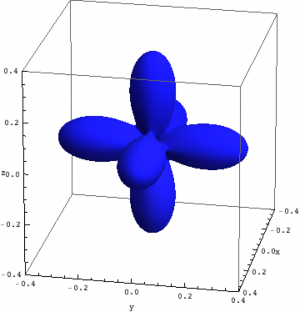 | 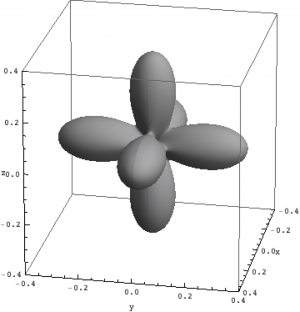 | 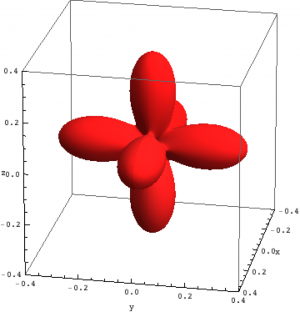 |
| Hole density of the $3d$ Ni orbitals in NiO | ||
The plots can be seen in Fig. \ref{figNiODensityMatrix}. We show the hole density (identity matrix minus the density matrix) which has lobes towards the O atoms. Ni in NiO has a hole in the $x^2-y^2$ and in the $z^2$ orbital. The color represent the spin down, $S_z=0$ and spin up state of the lowest triplet.
The output to the screen is:
- Density_matrix_plots_Mathematica.out
# <E> <S^2> <L^2> <J^2> <S_x^3d> <L_x^3d> <S_y^3d> <L_y^3d> <S_z^3d> <L_z^3d> <l.s> <F[2]> <F[4]> <Neg^3d> <Nt2g^3d><Neg^Ld> <Nt2g^Ld><N^3d> 1 -3.503 1.999 12.000 15.095 -0.370 -0.115 -0.370 -0.115 -0.741 -0.230 -0.305 -1.042 -0.924 2.186 5.990 3.825 6.000 8.175 2 -3.395 1.999 12.000 15.160 -0.002 -0.000 -0.002 -0.000 -0.003 -0.001 -0.322 -1.043 -0.925 2.189 5.988 3.823 6.000 8.178 3 -3.286 1.999 12.000 15.211 0.369 0.113 0.369 0.113 0.737 0.227 -0.336 -1.043 -0.925 2.193 5.987 3.820 6.000 8.180 Mathematica 8.0 for Mac OS X x86 (64-bit) Copyright 1988-2011 Wolfram Research, Inc. Loaded the Quanty : Plot Tools Package - version 2016.4.13 Written by Maurits W. Haverkort